A study led by OHSU has taken the first step in demonstrating that functional egg cells can be produced from skin cells. While these results have garnered significant interest in the scientific community, they are currently considered a conceptual proof and require more detailed safety testing before clinical application.
This approach, which holds promise for couples struggling with infertility worldwide, currently offers only limited solutions for individuals facing a lack of healthy gametes. Although in vitro fertilization (IVF) remains the most helpful treatment, it is not sufficient for women with completely non-functional ovaries. This situation raises the possibility that different cells in the body can be reprogrammed into egg and sperm cells.
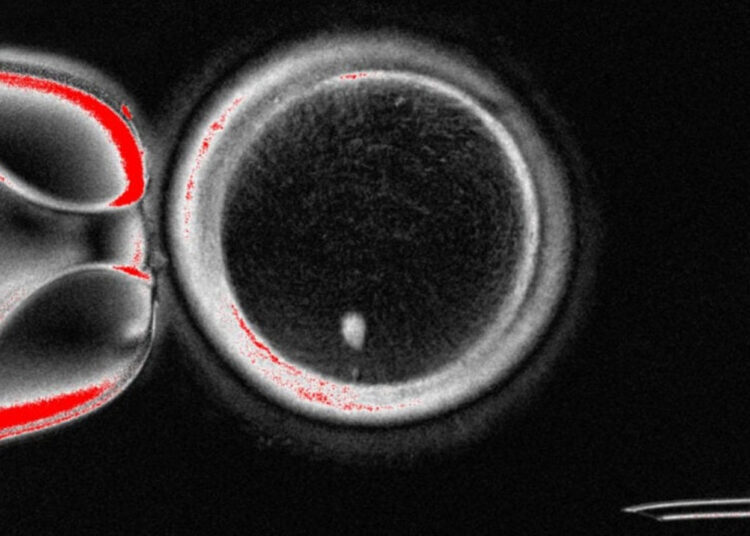
The team’s work demonstrated that the method called in vitro gametogenesis (IVG) could also be applicable to humans. Previous trials had shown success in mice; now, it is noted that this possibility could also be practically viable in humans for the first time. In the study, the nucleus of the egg was replaced with that of a skin cell to initiate a new process; this technique is based on somatic cell nuclear transfer (SCNT), which has been used in cloning studies before. However, the fundamental problem encountered here was the difference between cells with two sets of chromosomes in nature and cells with a single set.
To overcome this difference, a new method resembling meiosis was developed. The resulting cells reduced the two sets of chromosomes to a single set, aiming to create a chromosome arrangement close to that seen in natural egg and sperm cells. As a result, the researchers produced a total of 82 functional egg cells, and about 9% of them were fertilized. Some reached the blastocyst stage within five days, which is a critical step in the early developmental process of the embryo.
The blastocyst stage refers to the developmental phase associated with the embryo attaching to the uterus during IVF treatment. However, these results also pose some practical challenges: most of the blastocysts were found to carry chromosomal abnormalities, and some cells showed features different from natural eggs even if the chromosome count was correct. In particular, the cross-fertilization process that allows the mixing of chromosomes from the mother and father was missing; since this step is critical for genetic diversity, the likelihood that reprogrammed eggs will develop into a healthy fetus is currently low. The research was published in the journal Nature Communications.